The research arm of the U.S. Army has awarded Case Western Reserve University blood surrogate pioneer Anirban Sen Gupta a four-year, $2.5 million grant to advance and optimize his latest nanotechnology to stop bleeding from battlefield injuries.
The new technology devised by Sen Gupta and his team is called “SanguiStop.” It allows a clot-promoting enzyme called thrombin to be intravenously delivered in a targeted manner to a bleeding area—especially to the site of internal injuries.
Once there, the thrombin makes a specialized protein called fibrin—the body’s mesh-like substance critical to stanching the bleeding.
The technology could be especially helpful to treat soldiers who suffer from severe battlefield wounds, as well as patients who may have genetic or drug-induced defects in blood coagulation.
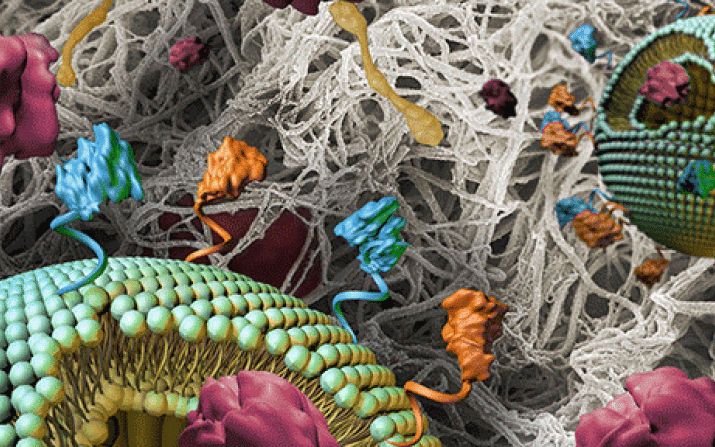
“Think of it like having concrete in place to build a dam and reduce flooding (in this case bleeding)—but you’ve got to deliver the concrete only where it’s needed, not everywhere in the bloodstream,” said Sen Gupta, the Leonard Case Jr. Professor of Engineering at the Case School of Engineering. “And that’s what we’re working on—using targeted phospholipid nanoparticles to get the thrombin where it needs to go.”
Phospholipids are important building blocks for the structure and function of living cells. They can also be assembled into nanoparticles 100-200 nanometers in size.
In this case, Sen Gupta and team made phospholipid nanoparticles with “homing molecules” on their surface that target them specifically to the injury site, once introduced into the bloodstream.
The new U.S. Army research funding will enable Sen Gupta and his team over the next four years to “make this technology reproducible, to optimize the dosage and confirm toxicity limits and immune risks,” he said.
New nanoparticle technology
When there is a bleeding injury, the human body naturally produces thrombin at high concentrations, specifically at the injury site. That process then aids in locally creating fibrin and coagulating blood.
This “thrombin burst” occurs via rapid reactions involving unique molecules in the blood called coagulation factors that assemble on the surface of clot-promoting blood cells, called platelets, gathering at the injury site.
However, the body’s natural ability to make thrombin at the injury site is compromised for soldiers who have suffered severe blood loss or in patients who have blood defects, affecting fibrin formation at the site, Sen Gupta said.
Further, thrombin cannot be intravenously administered to the body to treat this problem because it would cause “indiscriminate clotting all over,” Sen Gupta said.
“So, instead, we have packaged thrombin within a nanoparticle carrier that specifically targets to the bleeding site and then releases the thrombin at the site to make fibrin where needed,” he said.
Sen Gupta and collaborators had been exploring this approach since the past year, he said. A feasibility study published recently in ACS Nano, a journal of the American Chemical Society, showed how it could be successful.
Focused on stopping the bleeding
Sen Gupta has partnered with collaborators for the last decade to advance cutting-edge research in synthetic blood surrogates, focusing especially on developing artificial platelet systems.
They also work on therapeutic technologies for hemostasis (stopping bleeding), thrombolysis (breaking harmful blood clots) and inflammation (numerous blood cell-related pathologies).
They have also developed a hand-held medical device to quickly assess a wounded soldier’s critical clotting issues and other blood conditions in the battlefield.
In 2016, he also co-founded Haima Therapeutics, a biotechnology company focused on bleeding-control technologies.
For more information, contact Mike Scott at mike.scott@case.edu.
This article was originally published Aug. 30, 2022, in The Daily.